Browse by Categories
-
Top Searching Keywords
-
Automobiles & Motorcycles
More CategoriesTop Searching Keywords
-
Top Searching Keywords
-
Top Searching Keywords
-
Consumer Electronics
More CategoriesTop Searching Keywords
-
Electrical Equipment
More Categories -
Top Searching Keywords
-
Top Searching Keywords
-
Health & Medical
More CategoriesTop Searching Keywords
-
Home & Garden
More CategoriesTop Searching Keywords
-
Lights & Lighting
More CategoriesTop Searching Keywords
-
Manufacturing Machinery
More Categories -
Minerals & Metallurgy
More CategoriesTop Searching Keywords
-
Packaging & Paper
More CategoriesTop Searching Keywords
-
Personal Care
More CategoriesTop Searching Keywords
-
-
- Agriculture
- Home & Garden
- Apparel
- Home Appliances
- Automobiles & Motorcycles
- Lights & Lighting
- Business Services
- Luggage, Bags & Cases
- Chemicals
- Manufacturing & Processing Machinery
- Computer Hardware & Software
- Measurement & Analysis Instruments
- Construction & Real Estate
- Minerals & Metallurgy
- Consumer Electronics
- Office & School Supplies
- Electrical Equipment & Supplies
- Packaging & Paper
- Electronic Components & Supplies
- Personal Care
- Energy
- Printing & Publishing
- Environment
- Rubber & Plastics
- Excess Inventory
- Security & Protection
- Fashion Accessories
- Service Equipment
- Food & Beverage
- Shoes & Accessories
- Furniture & Furnishings
- Sports & Entertainment
- General Industrial Equipment
- Telecommunications
- General Mechanical Components
- Textiles & Leather Products
- Gifts & Crafts
- Timepieces, Jewelry, Eyewear
- Hardware
- Tools
- Health & Medical
- Home >
- Products >
- Health & Medical >
- Physical Therapy Equipment (248)
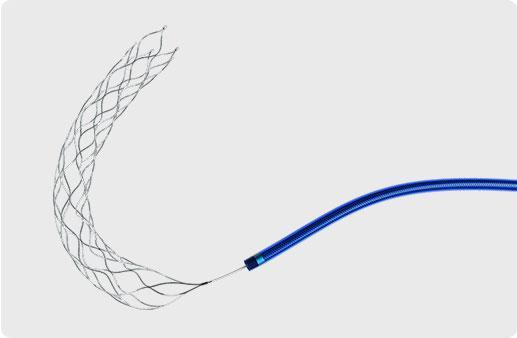
There are 7 clot retrieval device from 1 suppliers on EC21.com
Related Searches : medical equipment , therapy , therapy equipment , suction cupping , medical , cupping therapy